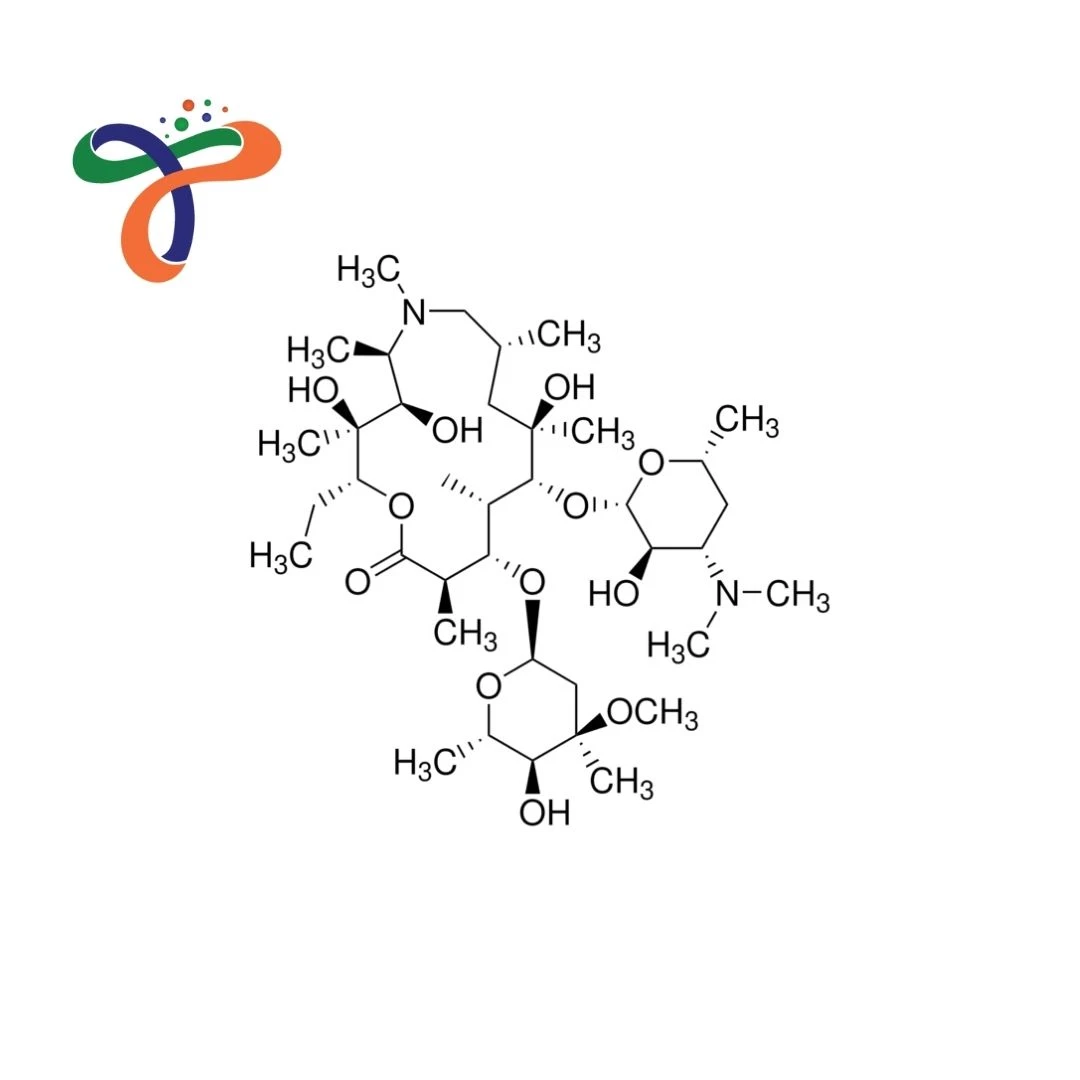
Azithromycin Anhydrous | 83905-01-5
Cas No :
83905-01-5
Form :
Solid
Molecular Weight :
748.99 G/Mol
Molecular Formula :
C38H72N2O12
Melting Point :
125 °C
Boiling Point :
260 °C
Solubility :
Sparingly Soluble In Water; Soluble In Methanol And Ethanol
Flash Point :
230 °C
Description :
Overview of Azithromycin Anhydrous
Azithromycin Anhydrous is a broad-spectrum macrolide antibiotic widely used in the treatment of bacterial infections affecting the respiratory tract, skin, soft tissues, and sexually transmitted infections. It works by inhibiting bacterial protein synthesis, thereby preventing the growth and replication of susceptible microorganisms.
Azithromycin Anhydrous appears as a white to off-white crystalline powder and is the water-free form of Azithromycin, offering improved stability for pharmaceutical formulations. It exhibits good bioavailability, high potency at low doses, and compatibility with various dosage forms such as tablets, capsules, and oral suspensions.
ChemicalBull supplies pharma-grade Azithromycin Anhydrous with high purity, controlled particle size, and consistent batch quality suitable for regulated pharmaceutical manufacturing. Each shipment is supported with complete documentation, including MSDS, COA, and technical data sheets, ensuring compliance with pharmaceutical industry requirements.
As a trusted chemical supplier and reliable distributor, ChemicalBull ensures dependable availability of Azithromycin Anhydrous for pharmaceutical manufacturers. You may also like Erythromycin, Clarithromycin, Amoxicillin, and Pharmaceutical APIs for related antibacterial applications.
Applications of Azithromycin Anhydrous
Pharmaceutical Formulations
-
Used in antibiotic tablets and capsules
-
Applied in oral suspension formulations
-
Effective against a wide range of bacterial infections
Hospital & Clinical Use
-
Used in the treatment of respiratory and skin infections
-
Applied to sexually transmitted infection therapies
-
Widely prescribed in acute and chronic bacterial infections
Generic Drug Manufacturing
-
Used in branded and generic antibiotic products
-
Suitable for large-scale pharmaceutical production
-
Ensures consistent potency and stability
Pharmaceutical Research & Development
-
Used as a reference standard in antibiotic research
-
Applied in bioequivalence and formulation studies
-
Suitable for controlled pharmaceutical laboratories
Safety & Handling Guidelines
-
Handle using gloves and appropriate protective equipment
-
Avoid inhalation of dust
-
Use in well-ventilated pharmaceutical facilities
-
Store in tightly sealed containers protected from moisture
-
Follow MSDS and standard pharmaceutical safety practices
Where to Buy Azithromycin Anhydrous?
Azithromycin Anhydrous Manufacturer
ChemicalBull supplies pharmaceutical-grade Azithromycin Anhydrous suitable for antibiotic formulations and regulated pharmaceutical applications.
Azithromycin Anhydrous Supplier & Distributor
-
Bulk and customised pharmaceutical packaging options
-
Export-grade quality with COA, TDS & MSDS
-
Reliable supply across India and international markets
-
Trusted chemical supplier for antibiotic APIs
Azithromycin Anhydrous MSDS
The Azithromycin Anhydrous MSDS provides essential information on safe handling, storage conditions, exposure controls, stability, and emergency procedures. Always review the MSDS before bulk handling or pharmaceutical manufacturing use.
Frequently Asked Questions (FAQs)
-
What is Azithromycin Anhydrous mainly used for?
It is mainly used as an antibiotic for treating bacterial infections of the respiratory tract, skin, and soft tissues. -
What is the difference between Azithromycin Anhydrous and the hydrated forms?
The anhydrous form contains no water, offering improved stability for pharmaceutical formulations. -
Is Azithromycin Anhydrous suitable for pharmaceutical manufacturing?
Yes, pharma-grade material is widely used in regulated pharmaceutical production. -
Is Azithromycin Anhydrous water-soluble?
It has limited water solubility but is formulated effectively in solid and suspension dosage forms. -
Is safety documentation provided with the supply?
Yes, every shipment includes MSDS, COA, and complete technical documentation.