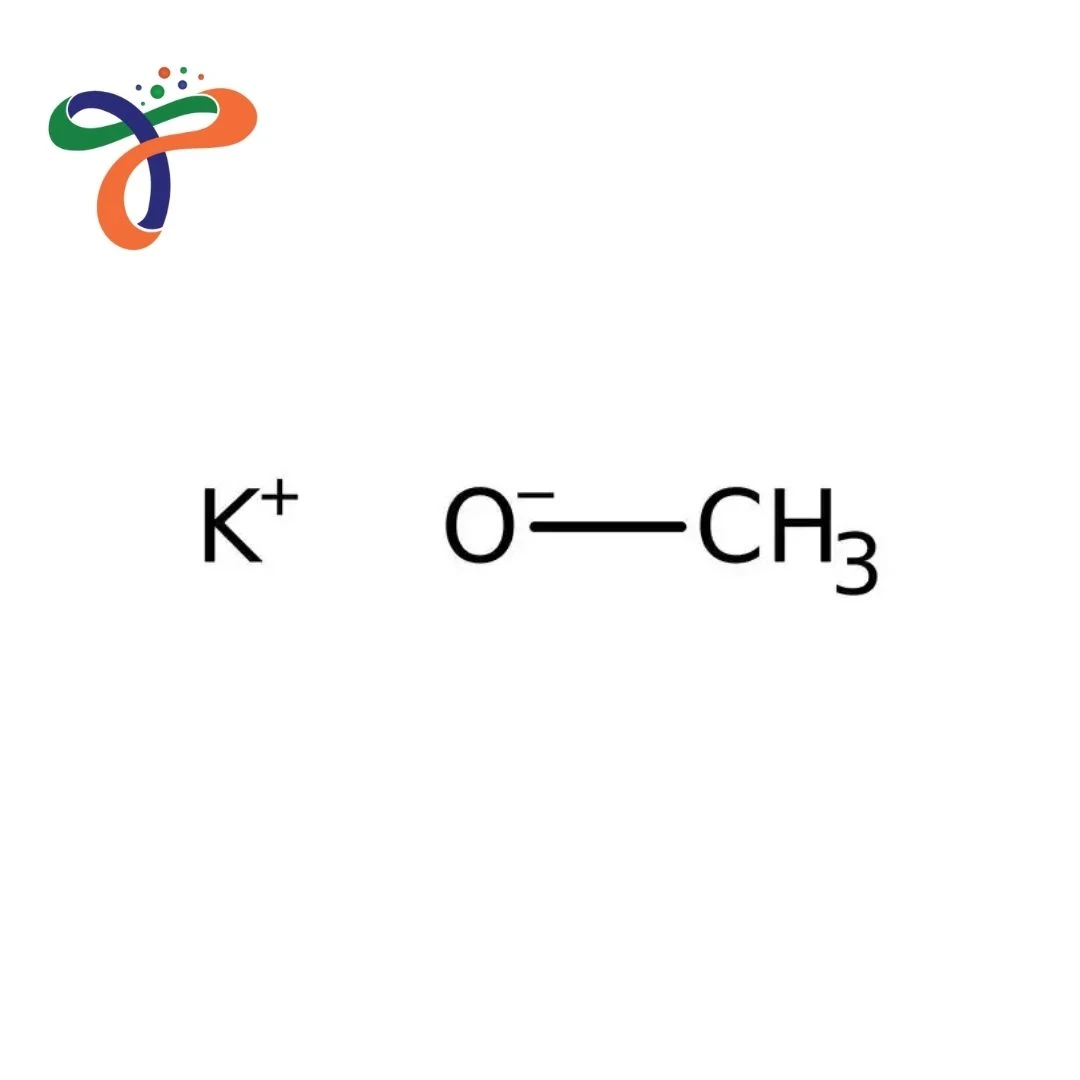
Potassium Methoxide | 865-33-8
Cas No :
865-33-8
Form :
Solid
Molecular Weight :
70.13 G/Mol
Molecular Formula :
CH3KO
Boiling Point :
360 °C
Melting Point :
60 °C
Flash Point :
60 °C
Solubility :
Soluble In Water; Soluble In Methanol And Other Alcohols; Hydrolyzes In Water
Description :
Overview of Potassium Methoxide
Potassium Methoxide is an extremely reactive alkoxide base that is used as a powerful base and catalyst for biodiesel production, organic synthesis, and the manufacturing of pharmaceuticals. It is renowned for its superior basicity in comparison to sodium methoxide and its excellent nucleophilicity. Potassium methoxide is frequently utilized in transesterification reactions, condensation, and as a reagent in various organic transformations.
The compound is an opaque white or yellowish powder or can be found as a solution in methanol. Its solid nature and high Reactivity make it among the most potent bases that are not nucleophilic that are available for organic syntheses.
Similar products are Sodium Methoxide, Potassium Ethoxide, Potassium Tert-Butoxide, and many other alkoxide bases utilized in industrial and organic synthesis applications.
Applications of Potassium Methoxide
Organic Synthesis
- It is used in Claisen condensation reactions
- Used for Dieckmann's cycleization
- In aldol condensations
- Utilized for transesterification reactions
- It is used to prepare carbanions and enolates
- Used in Michael Addition reactions
- Utilized in the production of pharmaceutical intermediates
- Used in the synthesis of heterocyclic compounds
Biodiesel Production
- Utilized as a catalyst for the transesterification of triglycerides.
- It is used to convert biodiesel from vegetable oils
- Utilized in the manufacture of methyl ester
- Utilized in the manufacture of renewable fuels
- It is used in an industrial biodiesel plant
- Used to improve biodiesel quality and yield
Pharmaceutical Synthesis
- Utilized in the manufacturing of active ingredients in pharmaceutical manufacturing
- Utilized in the production of pharmaceutical intermediates
- It is used to synthesize complex molecules
- Utilized for research in medicinal chemistry
- Used in developing drug candidates
- Used in the production of fine chemicals
Safety & Handling Guidelines
- EXTREMELY REACTIVE—pyrophoric in the presence of moisture
- Reacts violently with water, releasing flammable methanol vapor
- Use only under strictly anhydrous conditions
- Handle in an inert atmosphere (nitrogen or argon)
- Use appropriate PPE, including chemical-resistant gloves, face shield
- Store in tightly sealed containers under inert gas
- Keep away from moisture, water, acids, and oxidizers
- Have a dry chemical fire extinguisher available (never use water)
- Follow COA and MSDS instructions strictly
Where to Buy Potassium Methoxide?
Potassium Methoxide Manufacturer
ChemicalBull supplies high-purity Potassium Methoxide for pharmaceutical and industrial applications with appropriate safety packaging.
Potassium Methoxide Supplier & Distributor
- Available as powder or solution in methanol
- Multiple concentration options for solutions (25%, 30% in methanol)
- High-purity anhydrous grades
- Special packaging under an inert atmosphere
- COA, MSDS, and comprehensive safety documentation provided
MSDS
Includes comprehensive safety instructions, reactivity warnings, fire hazard information, and emergency procedures.
Frequently Asked Questions (FAQs)
-
What is Potassium Methoxide's structure?
Ionic compound consisting of potassium cation (K⁺) and methoxide anion (CH₃O⁻); formula: CH₃OK or KOCH₃. -
What is Potassium Methoxide CAS No?
CAS number: 865-33-8. -
What is Potassium Methoxide formula?
Molecular formula: CH₃OK with molecular weight 70.13 g/mol. -
What is Potassium Methoxide powder used for?
Used as a strong base in organic synthesis, a catalyst in transesterification and biodiesel production, and a reagent in pharmaceutical manufacturing.