Ammonia | 7664-41-7
Cas No :
7664-41-7
Form :
Gas
Molecular Weight :
17.030 G/Mol
Molecular Formula :
NH3
Boiling Point :
-33.34 °C
Flash Point :
-74 °C
Solubility :
Highly Soluble In Water
Description :
Overview of Ammonia
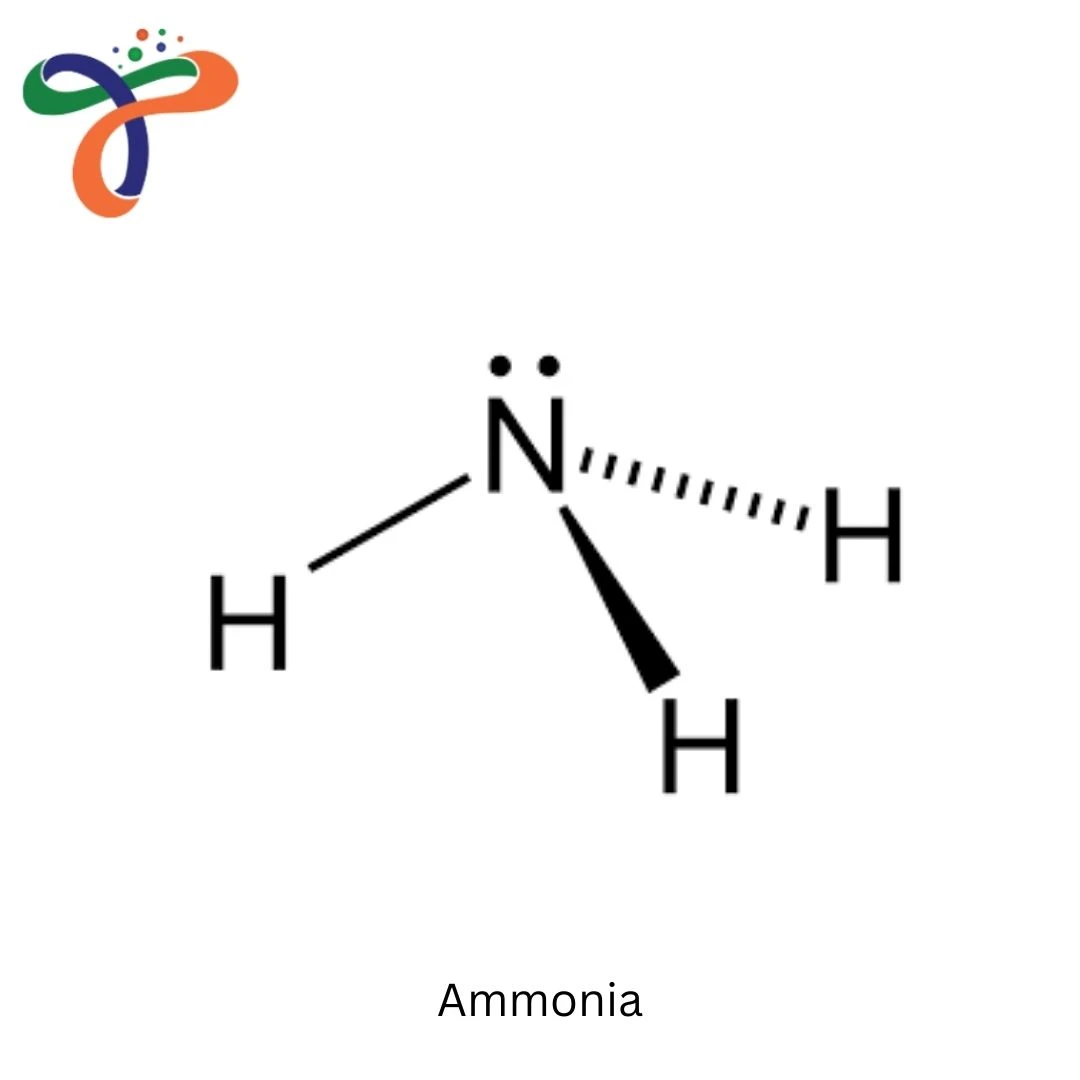
Ammonia (NH₃) is a colorless gas with pungent odor, essential for fertilizers, refrigerants, and industrial chemicals, known by its ammonia formula NH₃ or ammonia chemical formula NH₃. The product is available as anhydrous gas, aqueous solutions (20-30%), or in fertilizer formulations - where hydrogen gas combines with nitrogen to form ammonia through the Haber-Bosch process, providing percentage of nitrogen in ammonia at 82% by weight.
In simple terms, ammonia supplies critical nitrogen hydrogen ammonia nutrition for crops while serving as a cleaning agent, refrigerant, and chemical intermediate across multiple industries.
Applications of Ammonia
Nitrogen Fertilizer Production
Ammonia is the primary nitrogen source for urea, ammonium nitrate, and ammonium sulfate fertilizers.
- Provides percentage of nitrogen in ammonia (82%) for crop nutrition.
- Essential for Fertilizer Industry manufacturing.
- Complements Rock Phosphate phosphorus programs.
Industrial Refrigeration
Ammonia (NH₃) is an efficient refrigerant used in cold storage and food processing.
- It has a high latent heat capacity and is considered environmentally safe.
- Used in Agriculture Industry cold chains.
- Works with Gibberellic Acid crop storage systems.
Chemical Manufacturing
Hydrogen gas reacts with nitrogen to produce ammonia, which is then used as a raw material for plastics, explosives, and pharmaceuticals.
- Ammonia is a key ingredient in making nylon, acrylics, and hydrogen cyanide.
- Pairs with Cyanamide nitrogen chemistry.
- Supports Tributyltin Oxide industrial processes.
Cleaning & Water Treatment
Aqueous ammonia solutions are used in household cleaners and for adjusting pH levels.
- Disinfectant in Temephos vector control programs.
- Essential for Agriculture Industry sanitation.
Safety & Handling Guidelines
Always use strict safety precautions when handling ammonia, just as you would with other compressed gases and corrosive chemicals.
- Store ammonia in approved pressure cylinders or tanks, and keep them in cool, well-ventilated areas.
- Wear full protective gear, including chemical-resistant gloves, goggles, a face shield, and an SCBA respirator.
- Keep ammonia away from acids, oxidizers, copper, mercury, and anything that could ignite it.
- Make sure emergency eyewash stations, safety showers, and dilute acid for neutralization are available.
- Refer to the Material Safety Data Sheet (MSDS) for detailed information on toxicity and how to handle spills.
Ammonia MSDS
The MSDS for ammonia gives detailed information about its ammonia chemical formula, percentage of nitrogen in ammonia, toxicity profile, flammability limits, safe handling under pressure, and emergency medical treatment. This information is critical for fertilizer plants, refrigeration technicians, and chemical handlers.
ChemicalBull provides the MSDS, ammonia specifications, and handling instructions with every order.
Ammonia Manufacturer, Supplier & Distributor
Ammonia Manufacturer
Ammonia is manufactured through hydrogen gas combines with nitrogen to form ammonia (Haber-Bosch process) for fertilizer, refrigeration, and chemical industries.
Ammonia Supplier & Distributor
ChemicalBull is a trusted manufacturer, supplier, and distributor of ammonia. You can find it at chemicalbull in anhydrous gas cylinders, aqueous solutions, and fertilizer grades.
- Industrial grade (99.5%+ purity) meeting ammonia chemical formula NH₃ specifications.
- Ammonia is available in bulk tankers, cylinders, and drums to suit any application size.
- Every order includes full documentation, such as a Certificate of Analysis (COA).
- With 35+ years experience from the home page.
Frequently Asked Questions (FAQs)
-
What is the formula of ammonia?
The formula of ammonia is NH₃, also known as the ammonia formula or ammonia chemical formula, containing one nitrogen atom bonded to three hydrogen atoms. -
What is the percentage of nitrogen in ammonia?
Percentage of nitrogen in ammonia is approximately 82% by weight (14/17 atomic mass ratio), making it the most concentrated nitrogen source for fertilizers. -
How does hydrogen gas combines with nitrogen to form ammonia?
Hydrogen gas combines with nitrogen to form ammonia through the Haber-Bosch process: N₂ + 3H₂ ⇌ 2NH₃ at 200-300 atm and 400-500°C with iron catalyst. -
Is ammonia safe for fertilizer use?
Anhydrous ammonia needs special equipment, while aqueous ammonia solutions are safer for direct soil use and fertigation systems. -
What industries use ammonia most?
About 80% of ammonia is used in fertilizer production. It is also important in refrigeration, chemical manufacturing, cleaning, and water treatment industries.