Sodium Methoxide | 124-41-4
Cas No :
124-41-4
Form :
Solid
Molecular Weight :
54.02 G/Mol
Molecular Formula :
CH3NAO
Melting Point :
60 °C
Boiling Point :
125 °C
Solubility :
Soluble In Water And Polar Organic Solvents; Hydrolyzes In Water.
Flash Point :
125 °C
Description :
Overview of Sodium Methoxide
Sodium methoxide is an industrially reactive alkoxide chemical that is extensively employed in the manufacturing process where high basicity, rapid reaction kinetics, and dry conditions are needed. It is typically sold as a white to somewhat yellow solid or a methanol solution to ensure controlled handling in industrial processes. Sodium Methoxide is admired for its powerful nucleophilic properties, as well as its predictable reaction behavior and consistent results in chemical synthesis for industrial use.
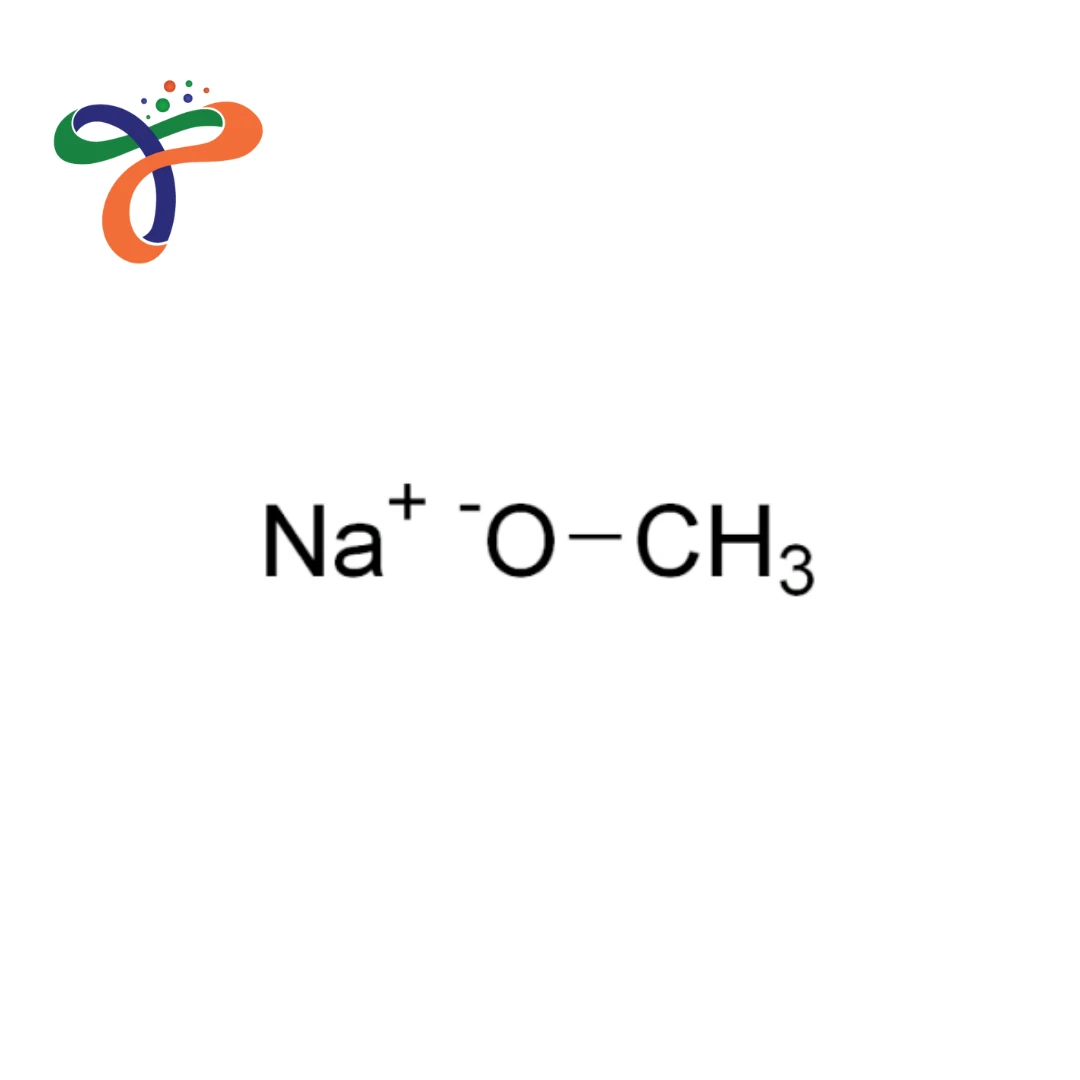
Sodium methoxide formula is CH₃ONa, and due to its strong basic nature, it is extensively used in industrial chemical manufacturing, specialty synthesis, biodiesel production, and other controlled processing applications where precise base-catalysed reactions are essential.
Applications of Sodium Methoxide
Chemical Manufacturing & Industrial Synthesis
It is extensively used in industrial chemical manufacturing as a catalyst and strong base for condensation, transesterification, or alkylation processes. It provides high efficiency in reaction and a consistent output of batches under controlled, dry conditions.
Biodiesel & Ester Production
Sodium Methoxide is extensively used as a catalyst in biodiesel manufacturing, where it enables efficient transesterification of oils and fats. It provides fast reaction rates and stable conversion efficiency in large-scale industrial biodiesel production.
Specialty Chemical & Process Chemistry
Sodium methoxide is utilized in special industrial chemistry, where solid base conditions are needed to create intermediates in chemical reactions. It guarantees predictable pathways for reaction and stable processing for industrial equipment.
Industrial Catalyst Applications
Sodium Methoxide is also used as an industrial catalyst in various manufacturing processes where controlled reactivity, rapid conversion, and consistent chemical performance are critical.
Safety & Handling Guidelines
- Keep it in a cool, dry, well-ventilated and dry area
- Make sure containers are tightly sealed and shielded from water
- Reacts violently with water and moisture
- Avoid inhalation of vapours or dust
- Avoid contact with skin and eyes
- Use protective gloves, goggles, a face shield, and suitable industrial clothing
- Handle under controlled conditions with proper ventilation
- Dispose of residues and packaging as per regulatory guidelines
Where to Buy Sodium Methoxide?
Sodium Methoxide Manufacturer
Sodium Methoxide is manufactured for industrial applications where high purity, controlled reactivity, and consistent quality are required for chemical synthesis and process manufacturing.
Sodium Methoxide Supplier & Distributor
The sodium Methoxide is available in solution or solid form for industrial usage. Being an industrial chemical supplier and distributor Chemicalbull Pvt. Ltd. provides clients with dependable sources, safe logistics, and standard documents to meet industrial needs.
MSDS for Sodium Methoxide
The MSDS for Sodium Methoxide provides detailed information on hazards, safe handling practices, storage requirements, exposure controls, first-aid measures, and emergency response procedures. Always review the MSDS before industrial use.
Frequently Asked Questions (FAQs)
-
What is the chemical formula of Sodium Methoxide?
Sodium methoxide formula is CH₃ONa. -
What is the structure of Sodium Methoxide?
The sodium methoxide structure is comprised of the sodium cation that is joined to a methoxide anion (CH3O-), creating an extremely reactive ionic compound. -
Is sodium methoxide a strong base?
Yes, Sodium Methoxide is a strong base, commonly used in industrial processes that require high basicity and fast reaction kinetics. -
Is sodium methoxide toxic?
Sodium Methoxide is hazardous due to its corrosive nature and high reactivity. Exposure may cause burns, irritation, or respiratory issues. Strict industrial safety measures and PPE are required. -
What happens when sodium methoxide reacts with water?
When Sodium Methoxide reacts with water, it decomposes rapidly, producing methanol and sodium hydroxide, along with heat. This reaction can be hazardous and must be avoided during handling.