N-Butyl Chloride | 109-69-3
Cas No :
109-69-3
Form :
Liquid
Molecular Weight :
92.57
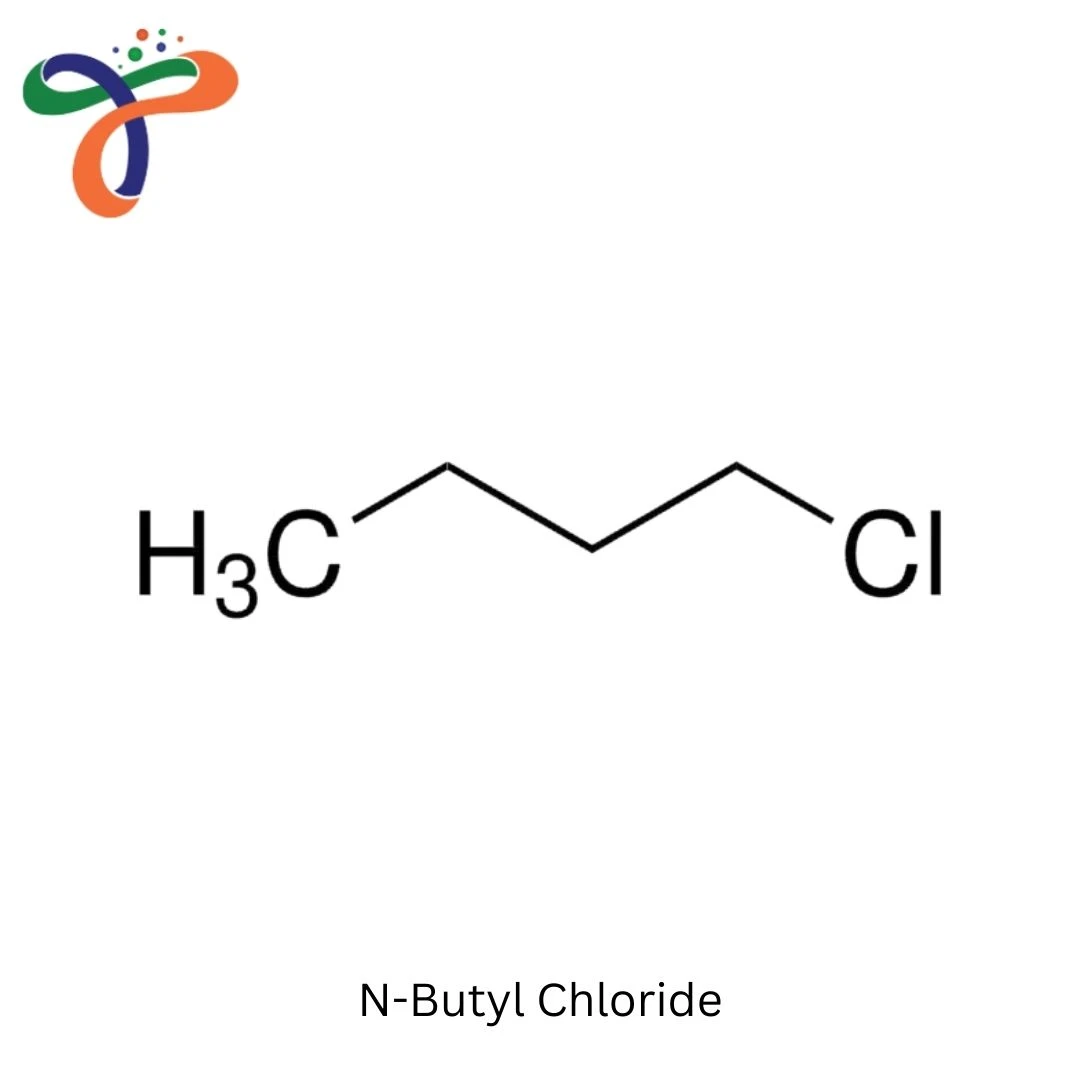
Molecular Formula :
C4H9CL
Melting Point :
123.1 °C
Boiling Point :
77 - 78 °C
Solubility :
Acetontrile (Slightly), Chloroform (Slightly), Ethyl Acetate (Slightly)
Flash Point :
12 °C
Description :
Overview of N-Butyl Chloride
N-Butyl Chloride (also called 1-Chlorobutane) is a non-coloured volatile organic compound that is widely utilised as an alkylating chemical and solvent for chemical manufacturing. It is highly valued for its role in evaporation-controlled processes and as a catalyst for the production of agricultural chemicals, pharmaceuticals, and speciality chemical compounds.
The n-butyl chloride structure consists of a straight-chain butyl group attached to a chlorine atom, allowing prediction of chemical reactions. The defined boiling point, the properties of the solvent, and its ability to be used in industrial and laboratory-scale reactions make it an incredibly versatile agent. Because of its high volatility, it is essential to handle and store it with care.
In formulations for industrial use, N-Butyl Chloride is commonly used along with other intermediates such as Monocalcium Phosphate Anhydrous, Monoethylene Glycol, and Morpholine, which are available from ChemicalBull and provide all chemical sources for various requirements in manufacturing.
Applications of N-Butyl Chloride
Chemical & Organic Synthesis
-
Used as an alkylating agent in organic reactions
-
Important intermediate in pharmaceutical and agrochemical synthesis
-
Supports the formation of quaternary ammonium compounds
Solvents & Industrial Processing
-
Used as a solvent in the processing of resins, oils, and fats processing
-
Helps in the extraction and purification steps
-
Preferred for reactions requiring controlled volatility
Pharmaceutical & Speciality Chemicals
-
Applied in the synthesis of active pharmaceutical intermediates
-
Used in fine chemical and speciality product manufacturing
-
Ensures consistent reaction performance
Research & Laboratory Use
-
Standard reagent in organic chemistry laboratories
-
Used in reaction mechanism studies and synthesis development
-
Often used with Morpholine and Monoethylene Glycol in multi-step processes
Safety & Handling Guidelines
-
Very flammable. Keep out of direct sparks, heat or sparks that ignite.
-
Make sure you wear goggles, gloves and wear appropriate clothing to protect yourself.
-
Beware of inhaling Vapours. Make sure you have adequate ventilation.
-
Keep it in a dry, cool and well-ventilated space
-
Make sure you follow the MSDS of n-butyl Chloride and safety precautions
Where to Buy N-Butyl Chloride?
N-Butyl Chloride Manufacturer
ChemicalBull supplies high-quality N-Butyl Chloride suitable for pharmaceutical, chemical, and industrial applications.
N-Butyl Chloride Supplier & Distributor
-
Bulk and customised packaging options available
-
Export-grade material with COA, TDS & MSDS
-
Reliable supply for manufacturers and research institutions
-
Explore related products: Monocalcium Phosphate Anhydrous, Monoethylene Glycol, Morpholine
N-Butyl Chloride MSDS
The n-butyl chloride MSDS provides detailed guidance on safe handling, storage conditions, health hazards, exposure limits, and emergency measures. Always consult the MSDS before industrial or laboratory use.
Frequently Asked Questions (FAQs)
-
What is N-Butyl Chloride used for?
It is used as an alkylating agent, a solvent, and an intermediate in the synthesis of pharmaceutical, agrochemical, and speciality chemicals. -
What is the boiling point of N-Butyl Chloride?
The boiling point of n-butyl chloride is approximately 78–79 °C, which contributes to its volatility and solvent behaviour. -
Is N-Butyl Chloride hazardous?
It is flammable and should be handled in accordance with the safety measures outlined in the MSDS.