Geosmin | 19700-21-1
Cas No :
19700-21-1
Synonyms :
Form :
Liquid
Molecular Weight :
182.30 G/Mol
Molecular Formula :
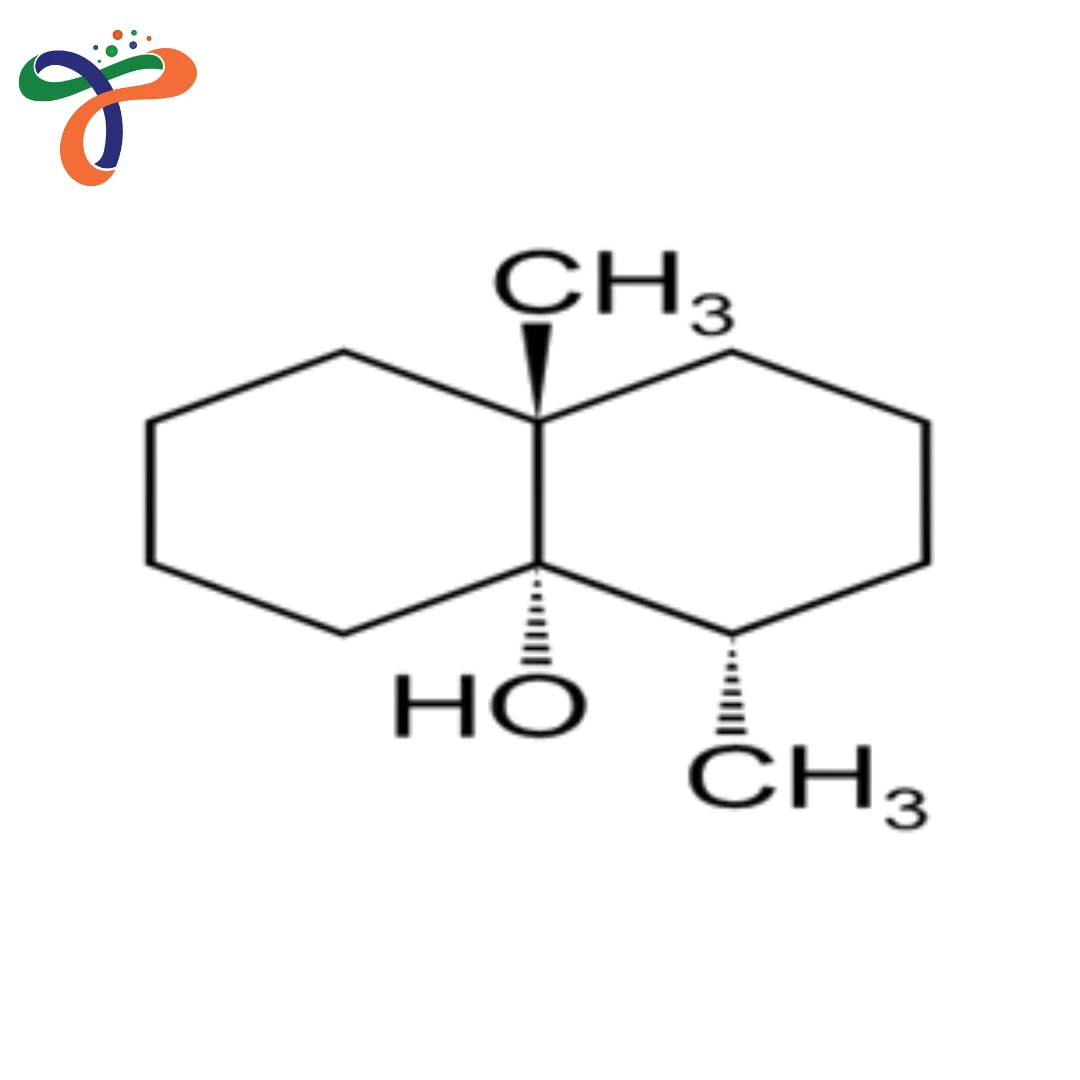
C₁₂H₂₂O
Melting Point :
–6 To –4 °C
Boiling Point :
270 °C
Solubility :
Slightly Soluble In Methanol, Chloroform , Practically Insoluble In Water
Flash Point :
110 °C
Description :
Overview of Geosmin
Geosmin is a colourless, oily, and naturally occurring irregular sesquiterpenoid alcohol best known for its distinctive earthy or musty odour, which is highly perceptible to humans even at extremely low concentrations. Found in soil, beetroots, and water, geosmin is responsible for the characteristic scent of rain on dry ground—petrichor—and contributes to taste and odour issues in drinking water and some foods. Its molecular formula is C₁₂H₂₂O, and it is a bicyclic compound produced through microbial metabolism, especially by the soil-dwelling bacteria Streptomyces and various cyanobacteria.
Applications of Geosmin
Flavour and Fragrance
Geosmin is occasionally added as a trace component in earthy or woody perfumery accords and some speciality flavours, especially when a natural soil, root, or beet note is desired in fine fragrances or gourmet foods. Related fragrance compounds like Vetiveryl Acetate and Methyl Ionone are often used alongside geosmin for complex olfactive effects.
Food and Beverages
Though generally unwanted for its earthy off-flavour, geosmin is a natural constituent of beets and can influence the sensory profile of vegetables, wine, and water. In product development, understanding geosmin's effect is crucial in root vegetable processing and water treatment. Analytical labs sometimes employ Dimethyl Sulfide as a comparative odorant in flavour research.
Drinking Water and Aquaculture
Geosmin is a key impurity responsible for taste and odour problems in drinking water, aquaculture, and fisheries. Its removal requires activated carbon treatment or oxidation. Monitoring geosmin is essential for water utilities and the fish industry, and is closely tied to the analysis of 2-methylisoborneol in water quality management.
Soil Science and Environmental Research
Geosmin serves as a biomarker for active soil bacteria and is used in ecological and geoscientific studies to track microbial activity. In environmental labs, it is analysed alongside compounds like Terpineol to assess soil health or the microbial impact on terrestrial and aquatic systems.
Industrial and Technical Uses
Due to its robust scent, geosmin is sometimes used as a sensory marker in gas leak detection, and trace-level addition is applied in agricultural product testing. Other odour standards, such as n-Butyl Mercaptan, provide context in industrial reference odorant libraries.
Safety & Handling Guidelines
-
Geosmin is considered non-toxic at typical environmental concentrations but should still be handled with care due to its potent aroma and volatility.
-
Wear gloves and avoid direct skin or mucous membrane contact to prevent transfer of its persistent odour.
-
Use in well-ventilated areas or fume hoods; geosmin is easily detected by smell, and its presence may linger in enclosed spaces.
-
Store in tightly closed containers at low temperatures (preferably -20°C) and away from strong acids or oxidisers.
-
For spills, absorb with inert material and ventilate the area until the odour dissipates; careful clean-up is essential as even trace amounts are highly impactful.
-
Avoid release to sewers or waterways in concentrated form, as it may contribute to taste and odour contamination.
-
Geosmin does not present acute toxicity concerns but may cause mild irritation if ingested or inhaled in significant quantities.
-
As with all analytical standards or aroma chemicals, practice good hygiene: wash hands after handling and do not eat, drink, or smoke in the handling area.
Where to Buy Geosmin?
Geosmin Manufacturer in India
ChemicalBull supplies high-purity geosmin for analytical, flavour, and fragrance uses, ensuring consistency and documentation for speciality product development.
Geosmin Distributor & Supplier in Vapi
ChemicalBull reliably distributes geosmin in small and bulk quantities for research, industrial, and flavor & fragrance applications.
Geosmin MSDS
For detailed guidelines on toxicity, handling, and environmental risk, request the geosmin Material Safety Data Sheet (MSDS) from ChemicalBull.
Frequently Asked Questions
-
What is geosmin used for?
Geosmin is used in flavour and fragrance design, analytical standards, and as a soil or microbial biomarker; it is also important in water and food quality management. -
Is geosmin safe?
Geosmin is not considered toxic at low concentrations but should be handled carefully to avoid persistent odour transfer and possible mild irritation. -
Why does geosmin smell like soil?
Geosmin is produced by soil bacteria such as Streptomyces and releases a distinct earthy odour, which humans readily detect after rain or disturbance of the soil.