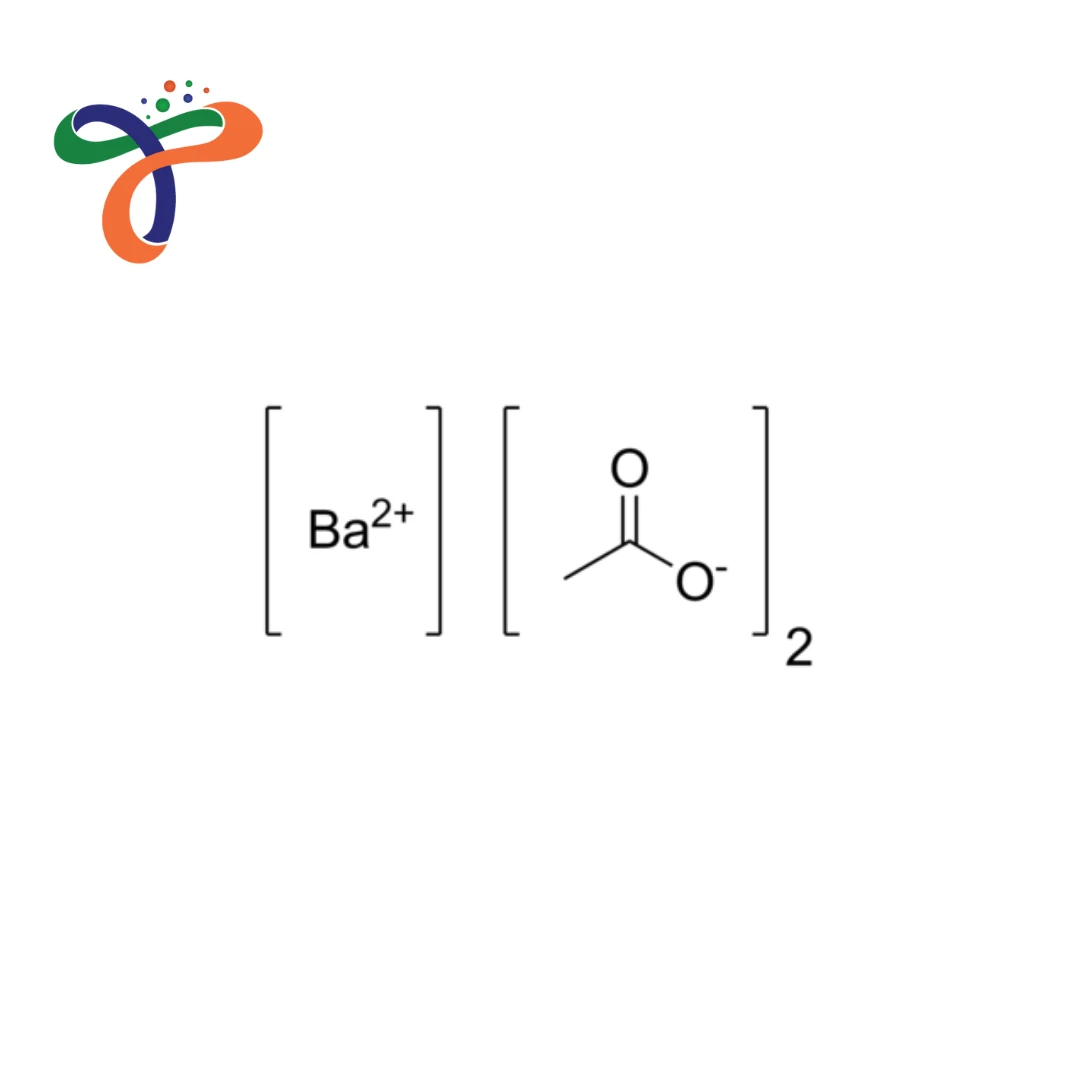Barium Acetate | 543-80-6
Cas No :
543-80-6
Form :
Solid
Molecular Weight :
255.42 G/Mol
Molecular Formula :
C4H6BAO4
Boiling Point :
1000 °C
Melting Point :
400 °C
Flash Point :
450 °C
Solubility :
Highly Soluble In Water
Description :
Overview of Barium Acetate
Barium Acetate is a barium salt made from acetic acid that is utilised as a chemical reagent catalyst, as well as in several industrial uses. It is renowned for its solubility in water and its role as a source of barium. Barium Acetate can be used for the manufacturing process, analytical chemistry, as well as specialised chemical syntheses.
The compound is crystals of white colour with hygroscopic characteristics. Much like the way Calcium Iodide as well as Calcium Sulphate are used as sources of calcium in a variety of applications, Barium Acetate provides a solution to barium that is soluble for certain applications in the analytical and industrial sectors.
Similar products are Barium Chloride, Barium Carbonate, Calcium Iodide, along with other alkaline and barium earth metallic salts.
An application that makes use of Barium Acetate
Chemical Manufacturing
- It is used as a catalyst for organic synthesis
- Used in the preparation of various barium compounds
- Utilised in the production of Acetate derivatives
- It is used in the polymer chemical industry
- Utilised in specific reactions of esterification
- It is used in a highly specialised chemical process
Analytical Chemistry
- Utilised as a reagent for analytic techniques
- Utilised for gravimetric analysis
- Utilised for precipitation reactions
- It is used for qualitative chemical analysis
- Standardisation solutions
- Utilised for research applications
Industrial Applications
- It is used in the textile industry for mordanting and in dyeing
- Used in the manufacture of pigments
- It is used for glass and ceramics manufacturing
- Applied in electronics manufacturing
- It is used to create specialised coatings
- It is used in certain formulations of pyrotechnics
Handling and Safety Guidelines
- Toxic--barium compounds are toxic
- Beware of ingestion, inhalation or skin contact
- Make sure you wear goggles, gloves, and protective clothes
- Use in areas that are well ventilated.
- Hygroscopic - store in tightly sealed containers
- Beware of acidic substances and other materials that are incompatible with HTML0.
- Be sure to follow COA and MSDS directions completely.
Where to Buy Barium Acetate?
Barium Acetate Manufacturer
ChemicalBull supplies high-purity Barium Acetate that meets analytical and industrial quality standards.
Barium Acetate Supplier & Distributor
- Available in various pack sizes for industrial and laboratory use
- Analytical and technical grades
- COA, MSDS, and safety documentation provided
MSDS
Includes detailed safety instructions, toxicity information, and handling guidelines.
Frequently Asked Questions (FAQs)
-
What is Barium Acetate formula?
Chemical formula: Ba(C₂H₃O₂)₂ or Ba(CH₃COO)₂ with molecular weight 255.42 g/mol. -
What are Barium Acetate uses?
Used in analytical chemistry, catalyst applications, manufacturing barium compounds, textile processing, and specialised industrial processes. -
What is Barium Acetate solubility?
Soluble in water (approximately 72 g/100 mL at 20°C); sparingly soluble in alcohol. -
What is Barium Acetate structure?
Consists of one barium cation (Ba²⁺) associated with two acetate anions (CH₃COO⁻). -
What is the formula for Barium Acetate?
Ba(CH₃COO)₂ or Ba(C₂H₃O₂)₂ · typically exists as monohydrate Ba(CH₃COO)₂·H₂O.