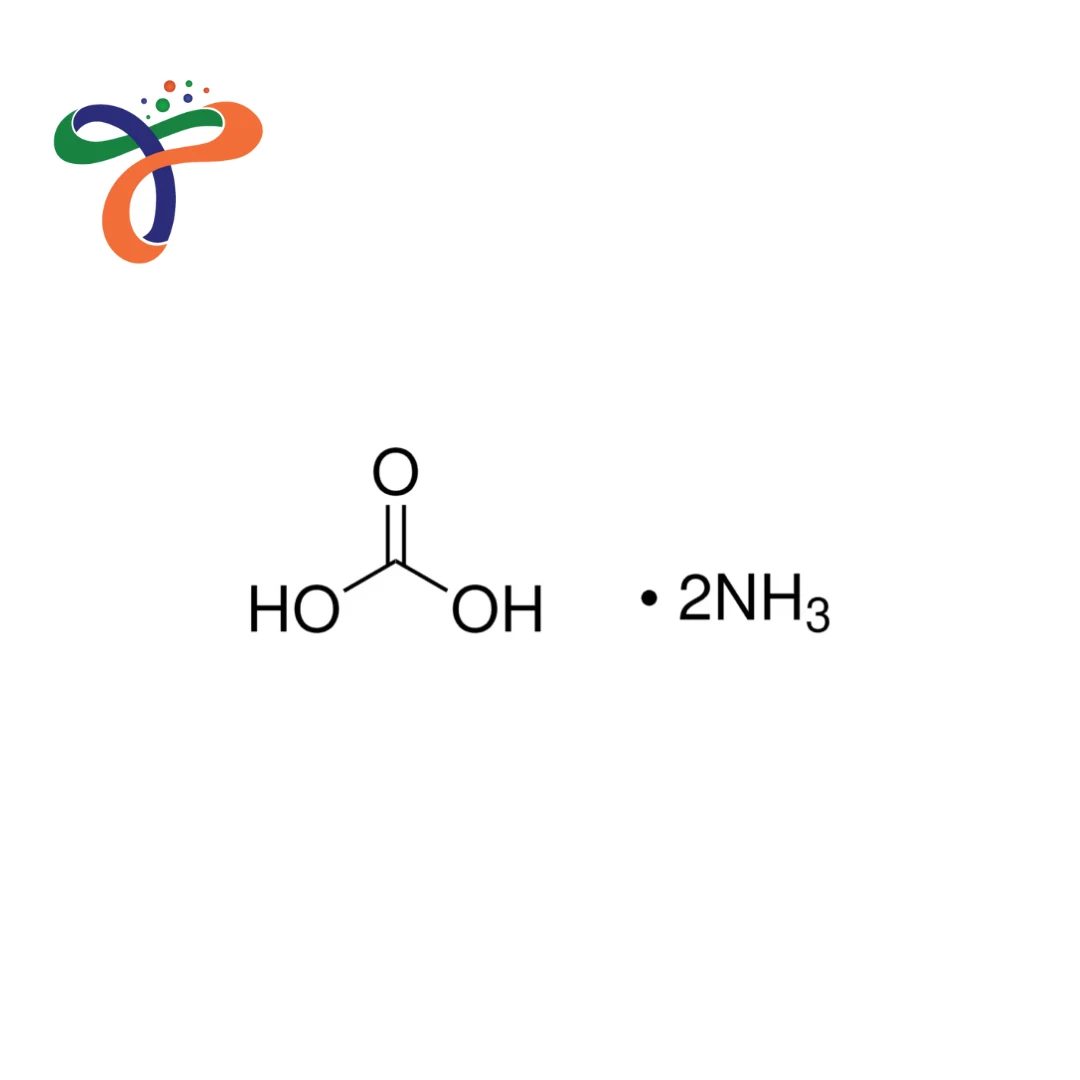
Ammonium Carbonate | 506-87-6
Cas No :
506-87-6
Form :
Powder
Molecular Formula :
CH8N2O3
Description :
Overview of Ammonium Carbonate
Ammonium Carbonate is an inorganic industrial salt that is employed in manufacturing processes in which controlled alkalinity, buffering, and the decomposition process that releases ammonia and carbon dioxide are needed. It's typically sold in the form of a white crystalline powder or granules, with a distinctive ammonia-like smell. Ammonium carbonate colour is usually white, which makes it suitable for use in industrial settings where a neat appearance and stable handling are desirable.
Ammonium carbonate decomposition happens when heated, which releases carbon dioxide. Carbon dioxide can be useful in processes that require control of gas releases or adjustment of pH in industrial processes.
Applications of Ammonium Carbonate
Industrial Chemical Processing
Ammonium Carbonate is utilised in industrial chemical processes where pH adjustment, buffering, and controlled alkalinity are needed. It provides stable control of reaction and stable manufacturing performance. It is typically used in conjunction with Ammonium Bicarbonate, depending on the needs of the process.
Pharmaceutical & Chemical Manufacturing Support
Ammonium Carbonate is used in industrial manufacturing where controlled alkalinity and buffering are required in processing steps. It supports consistent output quality and controlled formulation behaviour. It may be handled with Ammonium Hydroxide depending on industrial process needs.
Laboratory & Industrial Formulation Applications
Ammonium Carbonate can also be used in formulations for industrial use where stable chemical properties and controlled decomposition properties are needed. It allows for reliable batch handling and predictable processing results. It is possible to use it together with Ammonium Sulphite according to the specifications for formulation and application.
Industrial Utilities & Process Control
Ammonium Carbonate is used in industrial plants for process control applications where alkalinity adjustment and controlled chemical release are required. It supports stable operation performance in manufacturing environments.
Safety & Handling Guidelines
-
Keep in a cool, dry, well-ventilated and dry area
-
Make sure containers are tightly sealed to avoid the absorption of moisture
-
Beware of inhaling dust or ammonia fumes.
-
Avoid contact with the eyes and skin
-
Wear goggles, gloves and appropriate clothing for protection.
-
Keep away from heat sources due to the decomposition tendency
-
Maintain proper ventilation during handling and processing
-
Dispose of residues and packaging as per local regulations
Where to Buy Ammonium Carbonate?
Ammonium Carbonate Manufacturer
Ammonium Carbonate is manufactured for industrial applications where stable quality, controlled decomposition behaviour, and consistent performance are required for processing and formulation needs.
Ammonium Carbonate Supplier & Distributor
Ammonium Carbonate is supplied in bulk and packaged quantities for manufacturers requiring reliable quality and consistent supply. As an industrial Raw Material Distributor, Chemicalbull Pvt. Ltd. supports customers with dependable sourcing and standard documentation for industrial requirements.
MSDS for Ammonium Carbonate
The MSDS for Ammonium Carbonate provides detailed information regarding safe handling, storage conditions, and exposure control, First-aid measures, as well as emergency responses. Always read the MSDS prior to industrial use.
Frequently Asked Questions (FAQs)
-
What is ammonium carbonate used for?
Ammonium Carbonate is used in industrial chemical processing, food manufacturing (industrial baking), pharmaceutical processing support, and formulation applications requiring buffering and controalled alkalinity. -
Is NH4CO3 an acid or a base?
Ammonium carbonate behaves as a weak base in water due to carbonate ions producing alkaline conditions in solution. -
What is another name for ammonium carbonate?
Another name for ammonium carbonate is baker’s ammonia.