Triethylamine Hydrochloride | 554-68-7
Cas No :
554-68-7
Form :
Solid
Molecular Weight :
166,19 G/Mol
Molecular Formula :
C8H15NAO2
Melting Point :
> 300 °C
Description :
Overview of Triethylamine Hydrochloride
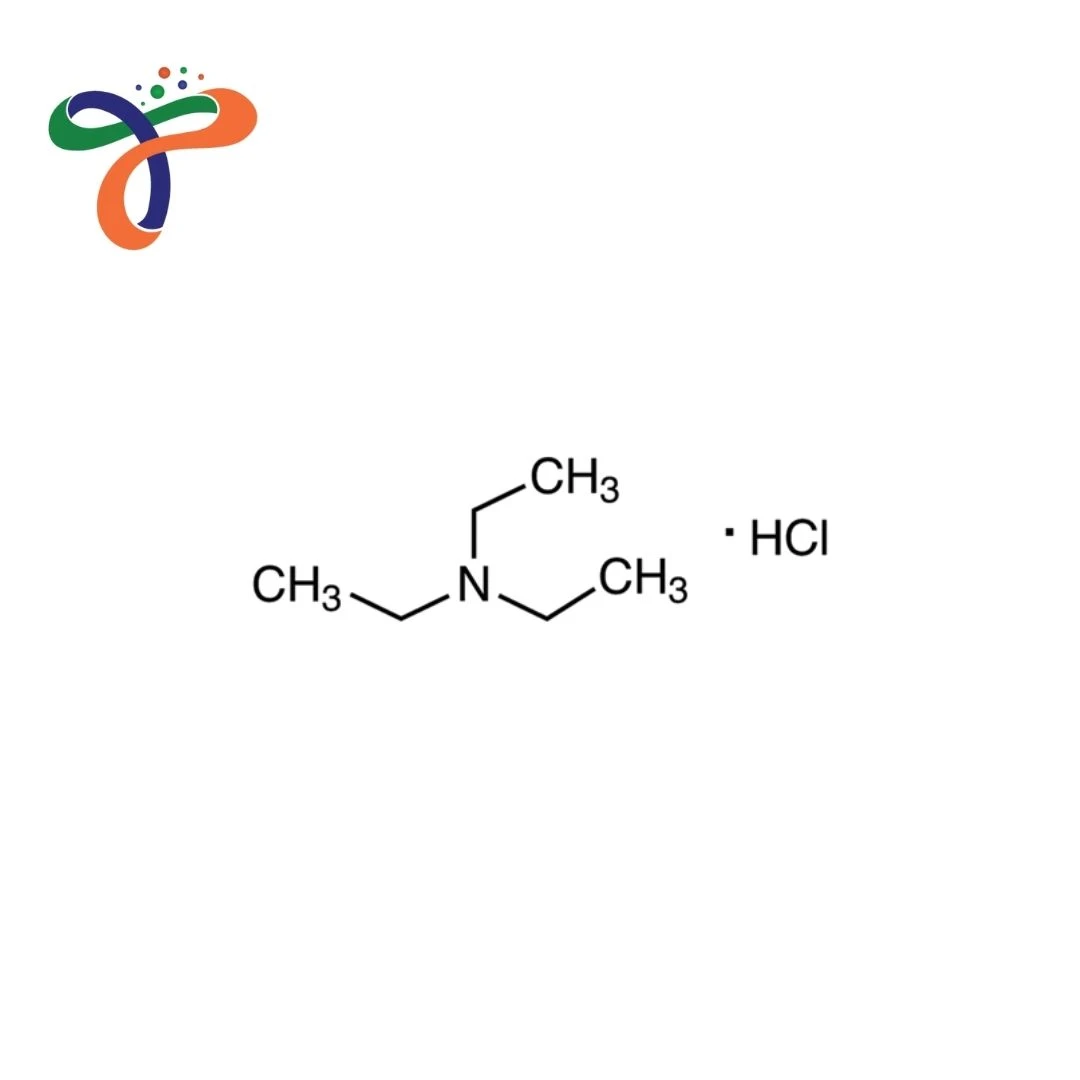
Triethylamine Hydrochloride is an ammonium quaternary salt that is widely employed in organic synthesis, catalysis, and chemical production. It is frequently combined with Mercury Sulfide or M-Xylene for industrial and laboratory reactions.
The chemical formula for triethylamine Hydrochloride can be found as C6H16ClN.
The structure is the triethylamine cation, which is paired with a chloride anion that can be used to provide effective neutralisation of acid-base within reactions.
Applications of Triethylamine Hydrochloride
Organic Synthesis
- It is utilised to neutralise acids in chemical reactions
- Supports salt formation for drug intermediates
Catalysis
- Serves as a catalyst in the esterification or amidation reaction
- Improves the reactivity of organic transformations
Industrial Chemical Manufacturing
- Applied in the production of fine chemicals and intermediates
- Used in pharmaceutical and speciality chemical formulations
Safety & Handling Guidelines
- Wear protective gloves, goggles, and clothing
- Avoid direct contact and inhalation
- Handle in ventilated areas or fume hoods
- Store away from moisture and strong oxidisers
- Follow MSDS and safety protocols
Where to Buy Triethylamine Hydrochloride?
Triethylamine Hydrochloride Manufacturer
ChemicalBull provides high-quality Triethylamine Hydrochloride for industrial and laboratory use.
Triethylamine Hydrochloride Supplier & Distributor
- Bulk and export-grade supply
- COA, TDS & MSDS available
- Reliable supply chain for manufacturers and researchers
Triethylamine Hydrochloride MSDS
MSDS contains information about safety, hazards, storage and emergency protocols.
Frequently Asked Questions (FAQs)
-
Which formula is for triethylamine Hydrochloride?
The chemical formula is C6H16ClN. -
What exactly is Triethylamine Hydrochloride utilised to solve?
It's used for organic synthesis as well as catalysis. -
Is Triethylamine Hydrochloride safe to handle?
It must be handled with care using PPE.