Ethyl Magnesium Bromide
Description :
Overview of Ethyl Magnesium Bromide
Ethyl Magnesium Bromide can be described as an organic reagent used for Grignard reactions as well as organic syntheses. It can be used in conjunction with Mercury Sulfide or M-Xylene for industrial chemical production.
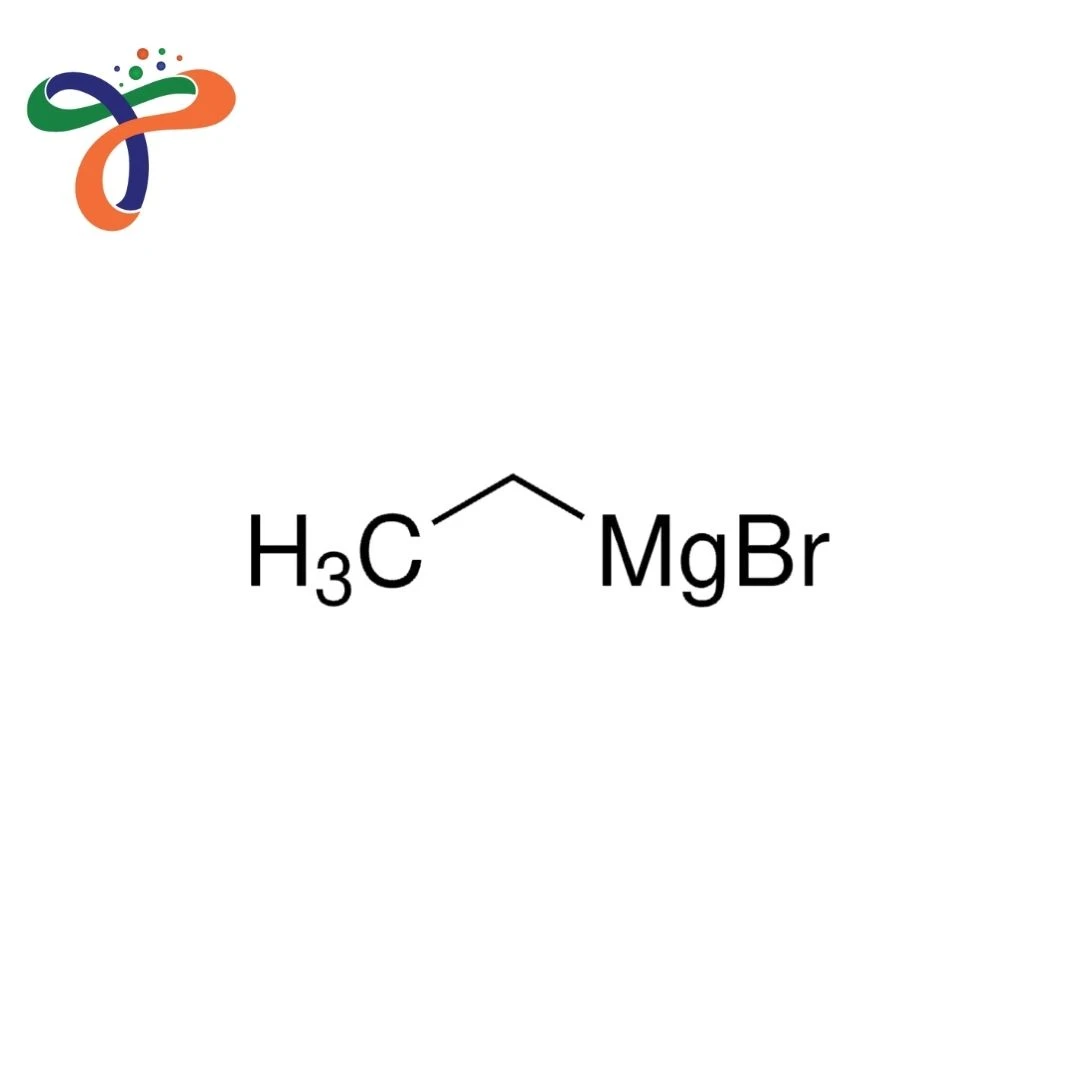
The chemical formula for Ethyl Magnesium bromide is C2H5MgBr. The structure is characterised by the magnesium atom that has been bonded with an ethyl atom and bromide ions, which provide high reactivity for carbon-carbon bonds.
Applications of Ethyl Magnesium Bromide
Grignard Reactions
- Carbon-carbon bonds are formed in organic syntheses
- Allows the creation of acids, alcohols, as well as intermediates
Industrial Chemical Manufacturing
- Used in the manufacturing intermediates for pharmaceuticals
- It is used in special chemical formulations
Organic Synthesis
- is a nucleophile in diverse chemical reactions
- It supports both industrial and laboratory synthesising processes
Safety & Handling Guidelines
- Protective gloves, goggles and wear appropriate clothing
- Avoid contact with the skin and inhalation
- Use in a non-toxic environment or with fume Hoods
- Storage should be in dry conditions and out of the reach of acidic environments
- Use MSDS and safety guidelines
Where to Buy Ethyl Magnesium Bromide?
Ethyl Magnesium Bromide Manufacturer
ChemicalBull supplies high-quality Ethyl Magnesium Bromide for industrial and laboratory use.
Ethyl Magnesium Bromide Supplier & Distributor
- Bulk and export-grade supply
- COA, TDS & MSDS available
- Reliable supply chain for manufacturers and researchers
Ethyl Magnesium Bromide MSDS
MSDS details hazards, safe handling, storage, and emergency procedures.
Frequently Asked Questions (FAQs)
-
What is the formula of Ethyl Magnesium Bromide?
The chemical formula is C₂H₅MgBr. -
What is Ethyl Magnesium Bromide used for?
It is used in Grignard reactions and organic synthesis. -
Is Ethyl Magnesium Bromide safe to handle?
It must be handled with care using PPE.